CeNS scientists develop new strategy for energy-efficient hydrogen production
Team L&M
Scientists have identified a new catalyst that can efficiently oxidise urea and lower the energy demand for hydrogen generation by urea-assisted water splitting thereby making way for improved production of the green fuel.
Understanding the importance of hydrogen energy in reversing climate change, the scientific community is intensifying efforts to revolutionize hydrogen production, a key player in the clean energy landscape. Electrolytic generation of hydrogen at cathode, while inherently clean and green, has been hampered by the energy demands of the oxygen evolution reaction at the anode (counter electrode). A viable solution emerges from replacing the oxygen evolution reaction with other anodic processes such as urea electro-oxidation reaction (UOR) possessing lesser overall cell potential. By adding urea to water, it has practically been shown to reduce the energy demand for electrochemical hydrogen production by about 30 per cent. This not only reduces the electrical energy input and hence, the cost for hydrogen generation from water but also holds a promise for remediating urea from wastewater in conjunction with energy generation while converting urea into nitrogen, carbonate, and water. Despite the potential advantages of this reaction, the catalysts developed so far not stable vulnerable to COx poisons (by-products of UOR) posing barriers to industry-scale implementation of this process.
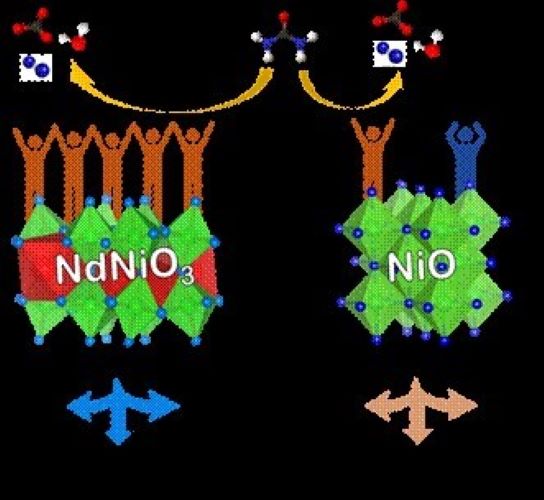
A team of scientists from Centre for Nano and Soft Matter Sciences (CeNS), Bengaluru – Nikhil N Rao, Dr Alex Chandraraj and Dr Neena S John, have demonstrated a non-noble metal catalyst, Ni3+-rich – Neodymium Nickelate (NdNiO3) with metallic conductivity that efficiently oxidizes urea, thereby lowering the energy demand for hydrogen generation by urea-assisted water splitting.
The investigation was taken up as part of an ongoing project to develop high-active and tolerant catalysts based on high-valent Ni-oxides for urea electrolysis, which is supported by the erstwhile Science and Engineering Research Board (SERB), now ANRF. The team used neodymium nickelate as an electrocatalyst for UOR, and using techniques such as X-ray absorption spectroscopy, electrochemical impedance spectroscopy, and Raman spectroscopy performed operando (under operating conditions), substantiated that the catalyst drives the reaction specifically through a ‘direct mechanism’. The direct mechanism exhibited by electrochemically activated neodymium nickelate stands out for its minimal catalyst degeneration and reconstruction, contrasting with the indirect mechanism requiring regeneration after each cycle of UOR that prevails in Ni2+-rich catalysts such as NiO. The catalyst has superior reaction kinetics (making the reaction faster), and enhanced stability during prolonged electrolysis, which are the attributes of a good electrocatalyst.
Towards addressing the challenge posed by COx poisons, which are known for deactivating UOR catalysts and compromising their long-term electrolysis durability, neodymium nickelate emerges as a promising solution. Its exceptional tolerance to COx poisons endows it with notable electrocatalytic stability. Computational calculations in collaboration with Dr. Moumita Mukherjee and Prof. Ayan Datta from Indian Association for the Cultivation of Science (IACS), Kolkata, validate the experimental findings.
Published in ACS Catalysis, a journal dedicated to publishing experimental and theoretical research on catalytic materials, this work could direct future studies aiming to enhance the number of NiOOH species and stabilize these species on Ni3+-rich substrates. The goal is to achieve improved performance with low mass loading of active Ni in the catalyst, marking a significant step towards sustainable and efficient hydrogen production.

